Have you ever looked at a chemical formula and wondered what it truly means, or how its parts connect? Well, it's almost like trying to understand a new language, isn't it? Knowing how atoms link up in something like CHCl3, which we often call chloroform, is pretty important. It tells us so much about what that substance can do, how it behaves, and even how it might feel or smell. Today, the 71st day of 2025, we're going to take a really good look at how we figure out these atomic arrangements.
Just like a good story needs clear sentences to make sense, a molecule needs a clear picture of its atoms and their electrons. That's where the Lewis dot structure comes in. It's a simple way to draw out all the valence electrons – those outermost electrons that do all the bonding work – and show how they are shared or transferred between atoms. This picture, you know, gives us a foundational glimpse into the molecule's personality, so to speak.
So, we're going to walk through the process of drawing the Lewis structure for CHCl3, step by step. It's not as tricky as it might seem, honestly. We'll explore why certain atoms go where they do, how many electrons are involved, and what that all means for the molecule's shape and characteristics. By the end, you'll have a much clearer idea of this common compound and, perhaps, a new appreciation for how molecular diagrams really communicate so much.
Table of Contents
- What is CHCl3, Anyway?
- Why Do We Draw Lewis Structures?
- Getting Ready: The Basics for CHCl3 Lewis Dot
- Beyond the Dots: Molecular Geometry and Polarity of CHCl3
- Common Questions About CHCl3 Lewis Dot
- What We've Learned About CHCl3
What is CHCl3, Anyway?
Well, before we jump into drawing dots and lines, it's pretty good to know what CHCl3 actually is. This chemical compound is more commonly known as chloroform. It's a colorless, dense liquid that, in the past, was used quite a bit as an anesthetic, though that's changed a lot over time. It also found uses as a solvent, which means it's good at dissolving other things, you know, like a very effective cleaner for certain substances.
Its chemical formula, CHCl3, tells us it has one carbon atom, one hydrogen atom, and three chlorine atoms. Just seeing the letters and numbers, however, doesn't really give us the full picture of how these atoms are arranged or what they're doing together. That's why we need a visual aid, something like a map for the atoms, to really get a sense of it. This map, or the Lewis structure, helps us understand its fundamental makeup, which is pretty cool.
Why Do We Draw Lewis Structures?
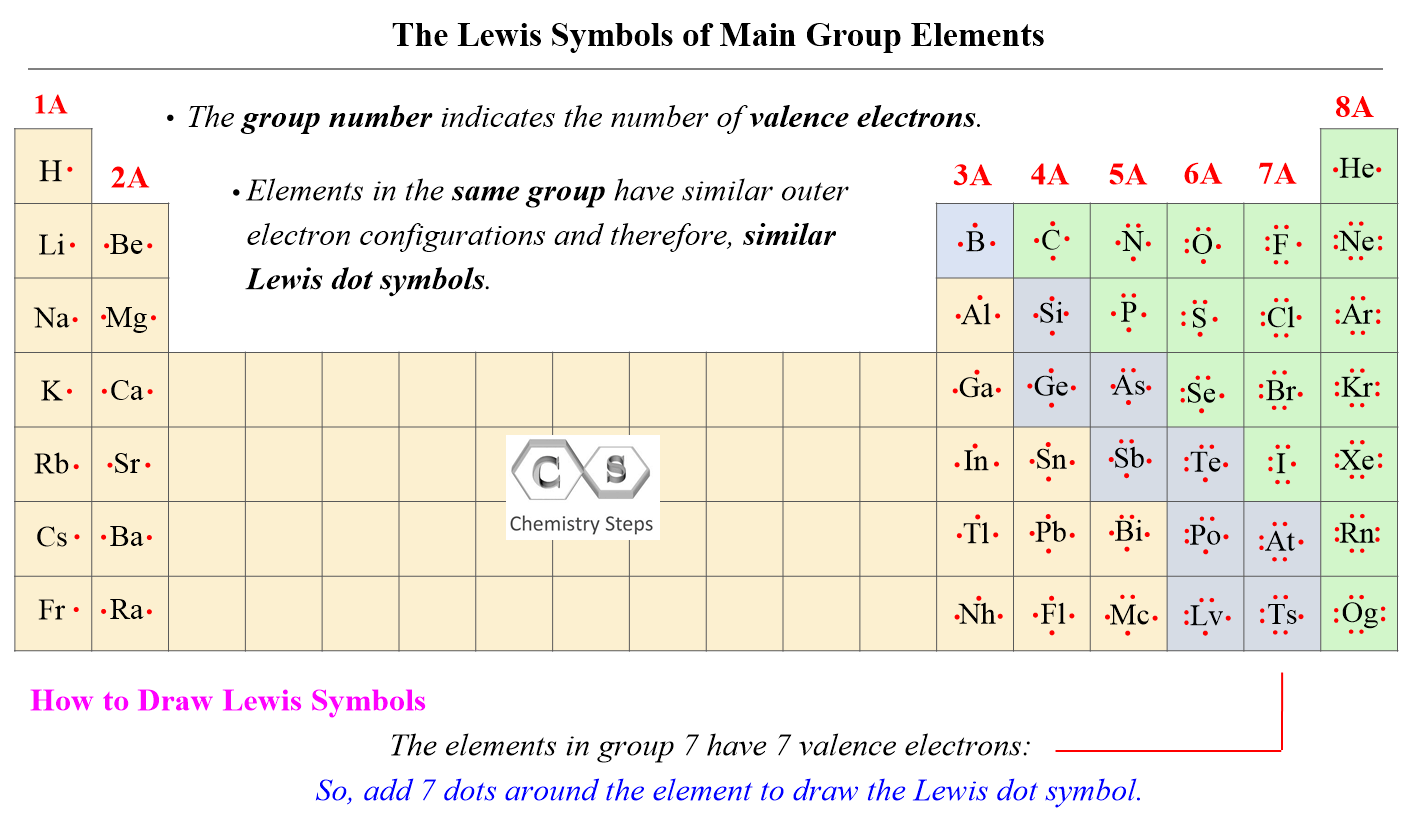
You might be wondering, "Why bother with all these dots and lines?" That's a fair question, and the answer is actually quite simple. Lewis structures are a foundational tool in chemistry, a bit like learning your ABCs before you can write a novel. They help us visualize the valence electrons, which are the electrons in the outermost shell of an atom. These are the electrons that are really involved in forming chemical bonds, so they're the ones that matter most for a molecule's behavior.
By showing these electrons, we can predict a lot about a molecule. For instance, we can figure out its shape, how reactive it might be, and whether it will mix with water or oil. It's a bit like how a clear message, like the philosophy of end-to-end encryption in WhatsApp, helps prevent misinterpretation; a clear Lewis structure helps us avoid misunderstanding a molecule's nature. Without this visual guide, understanding complex chemical reactions or properties would be, well, much harder, you know?
Getting Ready: The Basics for CHCl3 Lewis Dot
Alright, let's get down to the actual drawing. This isn't too hard, really, just a series of steps. Think of it like following a recipe; if you follow each instruction carefully, you'll end up with a pretty good result. We're going to build the CHCl3 Lewis dot structure piece by piece, ensuring we account for every single electron. This careful approach helps us avoid any missteps, which is pretty important when you're dealing with chemical structures.
The main idea here is to make sure every atom, if possible, achieves a stable electron configuration, often called an "octet." This means having eight valence electrons around it, either through sharing in bonds or as unshared lone pairs. Hydrogen is a bit different; it just needs two electrons. So, let's gather our tools, so to speak, and get started with the first step.
Step 1: Count All the Valence Electrons
The very first thing you need to do is figure out the total number of valence electrons available from all the atoms in the molecule. This is crucial because every single electron needs to be accounted for in your drawing. It's like making sure you have all the ingredients before you start cooking; you can't really begin without knowing what you're working with, can you?
For CHCl3, we have:
- Carbon (C) is in Group 14 of the periodic table, so it has 4 valence electrons.
- Hydrogen (H) is in Group 1, so it has 1 valence electron.
- Chlorine (Cl) is in Group 17 (halogens), so each chlorine atom has 7 valence electrons.
Now, let's add them up for the whole molecule:
- 1 Carbon atom x 4 electrons = 4 electrons
- 1 Hydrogen atom x 1 electron = 1 electron
- 3 Chlorine atoms x 7 electrons/atom = 21 electrons
Total valence electrons = 4 + 1 + 21 = 26 electrons. This number, 26, is what we'll be distributing throughout our structure. It's a pretty specific number, and we need to make sure we use all of them, no more, no less, which is really important for accuracy.
Step 2: Pick the Central Atom
After counting all those electrons, your next job is to decide which atom goes in the middle. The central atom is typically the least electronegative atom, or the one that can form the most bonds. Hydrogen, by the way, is almost never the central atom because it can only form one bond. It just doesn't have the capacity for more connections, you know?
In CHCl3, we have Carbon, Hydrogen, and Chlorine. Carbon is usually a good candidate for a central atom because it can form four bonds, which is quite a lot. Hydrogen, as we just mentioned, only forms one bond, and chlorine typically forms one bond but can sometimes expand its octet, though not in this particular simple structure. So, Carbon, in this case, is the clear choice for the central spot. It's kind of like the hub of a wheel, with other atoms radiating out from it.
Step 3: Connect Atoms with Single Bonds
With your central atom chosen, the next step is to connect all the other atoms to it using single bonds. Each single bond represents two shared electrons. This is the basic framework of your molecule, the skeleton, if you will. It's like drawing the main lines of a picture before you add all the details.
For CHCl3, you'll place the Carbon atom in the center. Then, you'll draw a single bond from the Carbon to the Hydrogen atom, and a single bond from the Carbon to each of the three Chlorine atoms. So, you'll have four single bonds radiating out from the central Carbon. This uses up a certain number of your total valence electrons, so we need to keep track.
- Number of bonds = 4 (C-H, C-Cl, C-Cl, C-Cl)
- Electrons used in bonds = 4 bonds x 2 electrons/bond = 8 electrons
Now, subtract these used electrons from your total: 26 (total) - 8 (used) = 18 electrons remaining. We still have quite a few electrons left to place, so, you know, we're not done yet.
Step 4: Place Lone Pairs on Outer Atoms
After forming those initial single bonds, your next task is to distribute the remaining electrons as lone pairs on the outer atoms. Remember, the goal is for each outer atom (except hydrogen, which only needs two electrons) to achieve an octet, meaning it should have eight electrons around it. Hydrogen, as we noted, is happy with just two, which it gets from its single bond.
In our CHCl3 structure, the outer atoms are Hydrogen and the three Chlorine atoms. Hydrogen already has its two electrons from the C-H bond, so it's all set. The Chlorine atoms, however, each have only two electrons from their single bond with Carbon. To reach an octet (8 electrons), each Chlorine atom needs 6 more electrons. These will be placed as three lone pairs on each Chlorine atom.
- Each Chlorine needs 6 electrons (3 lone pairs).
- Since there are 3 Chlorine atoms, total electrons for lone pairs on Chlorines = 3 atoms x 6 electrons/atom = 18 electrons.
Look at that! We had 18 electrons remaining from the previous step, and we just used all 18 of them on the outer Chlorine atoms. This is a pretty good sign that we're on the right track. We've used all 26 valence electrons, which is, you know, exactly what we wanted to do.
Step 5: Check the Central Atom and Octets
Now that all the electrons are placed, it's time for the final check. You need to make sure that every atom, especially the central atom, has a stable electron configuration. For most atoms, this means satisfying the octet rule – having eight electrons around them. Hydrogen, of course, is the exception, only needing two electrons.
Let's check our CHCl3 structure:
- Hydrogen (H): It has one single bond with Carbon, which means it has 2 electrons. Hydrogen is happy with 2 electrons. So, it's good to go.
- Chlorine (Cl): Each Chlorine atom has one single bond with Carbon (2 electrons) and three lone pairs (6 electrons). So, each Chlorine atom has a total of 2 + 6 = 8 electrons. All three Chlorines satisfy the octet rule, which is great.
- Carbon (C): The central Carbon atom has four single bonds (one to Hydrogen, three to Chlorines). Each bond contributes 2 electrons to the Carbon's count. So, Carbon has 4 bonds x 2 electrons/bond = 8 electrons. Carbon also satisfies the octet rule.
Since all atoms have achieved their stable electron configurations and we've used exactly 26 valence electrons, our Lewis dot structure for CHCl3 is complete and correct! It's actually a very satisfying feeling when everything just, you know, clicks into place like that.
Beyond the Dots: Molecular Geometry and Polarity of CHCl3
Drawing the Lewis structure is a fantastic start, but it's really just the beginning of understanding a molecule. The way atoms are arranged in three-dimensional space, and whether the molecule has a slight positive or negative end, tells us even more. This is where molecular geometry and polarity come into play, and they're directly related to that Lewis structure we just drew. It's like knowing the blueprint of a building helps you understand its overall shape and how it might interact with its surroundings.
The Shape of Things: VSEPR Theory
To figure out the actual 3D shape of a molecule, we use something called VSEPR theory, which stands for Valence Shell Electron Pair Repulsion theory. It's a bit of a mouthful, but the idea is pretty simple: electron pairs, whether they're in bonds or lone pairs, repel each other. They try to get as far away from each other as possible, which then dictates the molecule's shape. It's a very intuitive concept, actually, when you think about it.
For CHCl3, our central Carbon atom has four electron groups around it: one single bond to Hydrogen and three single bonds to Chlorine atoms. There are no lone pairs on the central Carbon. With four electron groups and no lone pairs on the central atom, the electron geometry and molecular geometry are both tetrahedral. This means the atoms are arranged like a pyramid with a triangular base, with bond angles of roughly 109.5 degrees. It's a very common shape in organic chemistry, so, you know, it's good to recognize it.
Is CHCl3 Polar or Nonpolar?
Once you know the shape, you can figure out if a molecule is polar or nonpolar. Polarity depends on two things: whether the individual bonds are polar, and whether those bond polarities cancel each other out due to the molecule's overall shape. A bond is polar if the two atoms sharing electrons have different electronegativities, meaning one pulls the shared electrons a bit more strongly than the other. This creates a slight charge separation, or a "dipole moment."
Let's look at CHCl3:
- C-H bond: The electronegativity difference between Carbon and Hydrogen is very small, so the C-H bond is considered essentially nonpolar.
- C-Cl bond: Chlorine is much more electronegative than Carbon. This means the C-Cl bonds are quite polar, with the electron density pulled towards the Chlorine atoms.
Now, consider the tetrahedral shape. While the C-H bond is nearly nonpolar, the three polar C-Cl bonds are arranged symmetrically around the carbon, but not in a way that their individual dipoles completely cancel each other out. Because the hydrogen atom is different from the three chlorine atoms, the molecule is not perfectly symmetrical in terms of its charge distribution. Therefore, CHCl3 has a net dipole moment, meaning it is a polar molecule. This characteristic, you know, affects how it interacts with other substances, like why it dissolves certain things but not others.
Common Questions About CHCl3 Lewis Dot
People often have a few questions when they're first learning about the Lewis structure of CHCl3. It's pretty normal to wonder about the specifics, especially when you're trying to get a clear picture in your head. Here are some of the most frequently asked ones, with answers that hopefully make things a bit clearer.
Is CHCl3 polar or nonpolar?
CHCl3 is a polar molecule. While it has a tetrahedral shape, the three polar C-Cl bonds and the one nearly nonpolar C-H bond do not cancel each other out perfectly due to the differing nature of the atoms attached to the central carbon. This creates an uneven distribution of electron density, giving the molecule a net dipole moment. So, it's definitely polar, which is, you know, an important property to remember.
What is the molecular geometry of CHCl3?
The molecular geometry of CHCl3 is tetrahedral. This is because the central carbon atom has four electron domains (four single bonds to other atoms: one H and three Cl) and no lone pairs. According to VSEPR theory, these four electron domains will arrange themselves as far apart as possible in three-dimensional space, leading to a tetrahedral shape with bond angles close to 109.5 degrees. It's a very common arrangement for molecules with four bonds and no lone pairs on the central atom.
How many lone pairs are in CHCl3?
In the Lewis structure of CHCl3, there are a total of nine lone pairs. Each of the three chlorine atoms has three lone pairs on it. The central carbon atom and the hydrogen atom do not have any lone pairs. So, you'll find all the lone pairs sitting on those chlorine atoms, which is pretty typical for halogens when they're bonded in this way.
What We've Learned About CHCl3
So, we've gone on a bit of a journey today, haven't we? From counting electrons to drawing lines and dots, we've really gotten to know the CHCl3 molecule, also known as chloroform, on a deeper level. We saw how the Lewis dot structure helps us visualize the valence electrons and how they are shared between atoms, which is, you know, the very foundation of understanding chemical bonding.
We walked through each step: figuring out the total valence electrons, picking the central atom, drawing those initial bonds, and then carefully placing all the remaining electrons as lone pairs. We made sure every atom, particularly the central carbon and the surrounding chlorines, achieved a stable electron configuration, usually an octet. This careful process is what gives us an accurate representation of the molecule's bonding.
Beyond just the dots, we also explored how this Lewis structure helps us predict the molecule's three-dimensional shape using VSEPR theory, revealing its tetrahedral geometry. And, by looking at the polarity of its individual bonds and its overall shape, we determined that CHCl3 is, in fact, a polar molecule. These insights are incredibly valuable because a molecule's structure and polarity truly dictate how it behaves in the world, from its boiling point to how it reacts with other substances.
Understanding these fundamental principles of molecular structure is, quite honestly, a skill that opens up so many doors in chemistry. It's a bit like learning the rules of a game; once you know them, you can play so much more effectively. So, why not try drawing a few more Lewis structures yourself? You could try something like methane (CH4) or water (H2O) to practice. You can learn more about molecular structure on our site, and perhaps even delve into the specifics of VSEPR theory explained for even more insights. Keep exploring, and you'll find that chemistry can be pretty fascinating!


Detail Author:
- Name : Mandy Bartoletti I
- Username : qlindgren
- Email : liliane.mckenzie@gmail.com
- Birthdate : 2004-08-14
- Address : 22610 Shields Viaduct South Evans, ID 88538
- Phone : 331-412-0899
- Company : Windler-Heaney
- Job : Healthcare Support Worker
- Bio : Deserunt mollitia qui et earum sit. Deserunt voluptate sit amet quibusdam a dignissimos. Sit provident molestiae pariatur commodi. Quas ratione quaerat unde magni in. Alias eos et dolore id.
Socials
linkedin:
- url : https://linkedin.com/in/boganc
- username : boganc
- bio : Dolor et totam quod delectus.
- followers : 4910
- following : 1488
twitter:
- url : https://twitter.com/caterina1107
- username : caterina1107
- bio : Est cumque similique reiciendis. Officia fugiat quo perferendis odit dolorem ducimus. Pariatur non nulla porro iure. Non dolorem eligendi et voluptatibus.
- followers : 2820
- following : 598
instagram:
- url : https://instagram.com/cbogan
- username : cbogan
- bio : Nam alias aut laborum et iure neque. Consequatur sed dolor culpa in.
- followers : 2475
- following : 2915

